In January 12, 2022, Simcere announces that the IND of a novel oral PRMT5 inhibitor (project number: SIM0272, product code: SCR-6920) in China for the treatment of advanced malignant tumors was accepted by NMPA.
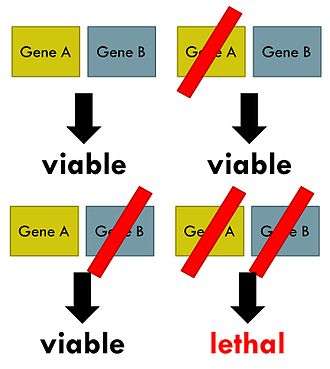
The candidate is independently developed by Simcere with potential mechanism of Synthetic lethality, where the combination of two genetic events results in cell death or death of an organism
The "synthetic lethality" theory has great significance in fighting cancer. Tumor cells usually have a lot more mutations and gene replication errors than normal cells do. Inhibition of the corresponding "synthetic lethal" paired genes can result in precise killing of certain tumor cells without harming normal cells.
The successful development of PARP inhibitors in the clinical treatment of ovarian cancer, breast cancer and other Breast Cancer gene (BRCA) mutant tumors is a fine example. The global PARP Inhibitor market is valued at 2178 million USD in 2020 and is expected to reach 16180 million USD by the end of 2026, growing at a CAGR of 32.8% during 2021-2026.
SCR-6920 is targeting another pair of synthetic lethal genes with great therapeutic potential: methylthioadenosine phosphorylase (MTAP)/ Protein arginine methyltransferases (PRMT).
In 2016, two articles published in the Science magazine were the first to report the "synthetic lethal" effect of inhibiting PRMT5 in MTAP-deficient tumors.
PRMT5 is overexpressed in various cancers such as lung cancer, breast cancer, gastric cancer, colorectal cancer, ovarian cancer, leukemia and lymphoma. It is associated with the progression and poor prognosis of many types of cancer, indicating its potential to be a promising anti-tumor target.
Preclinical studies have shown that SCR-6920 with its highly selectivity over PRMT5, potently inhibited tumor cell proliferation against various hematological and solid tumor cells in vitro. In multiple mouse CDX models, SCR-6920 alone has significantly inhibited tumor growth. On the other hand, SCR-6920 has demonstrated good cross-species pharmacokinetic properties, good safety profile and a relatively large therapeutic window.
Simcere is applying for approval of a multi-centered Phase I clinical study to evaluate the safety, tolerability, efficacy and pharmacokinetic characteristics of SCR-6920 in human. Once approved, Simcere will rapidly advance clinical research, in hope of bringing new clinical drug options for tumor patients in China soon.