The TASTE-Ⅱ Study, a multi-centered, randomized, double-blinded, and placebo-controlled clinical trial to validate the efficacy and safety of Sanbexin®, combining early endovascular recanalization in patients of Acute Ischemic Stroke (AIS), has recently achieved first patient enrollment (FPI) in Beijing Tiantan Hospital.
Led by Professor Yongjun Wang, Chairman of the Neurology Branch of the Chinese Medical Association and President of Beijing Tiantan Hospital, the TASTE-II study is designed to enroll 1300+ AIS patients in over 100 study centers across the country. The aim of the study is to evaluate the effect of Sanbexin® on facilitating neurological recovery in AIS patients receiving early endovascular recanalization (bridging therapy or direct endovascular therapy) within 24 hours of onset.
Stroke is the leading cause of disability and death in China. While endovascular therapy is considered the most effective solution to restore blood flow in ischemic stroke treatment, only about 50% of the patients can achieve favorable functional outcome (mRS 0-2) after 90 days of endovascular treatment. In order to reduce the disability rate and improve the functional prognosis of AIS patients, multi-target neuron protection has increasingly been valued as a promising strategy by the international neurology community.
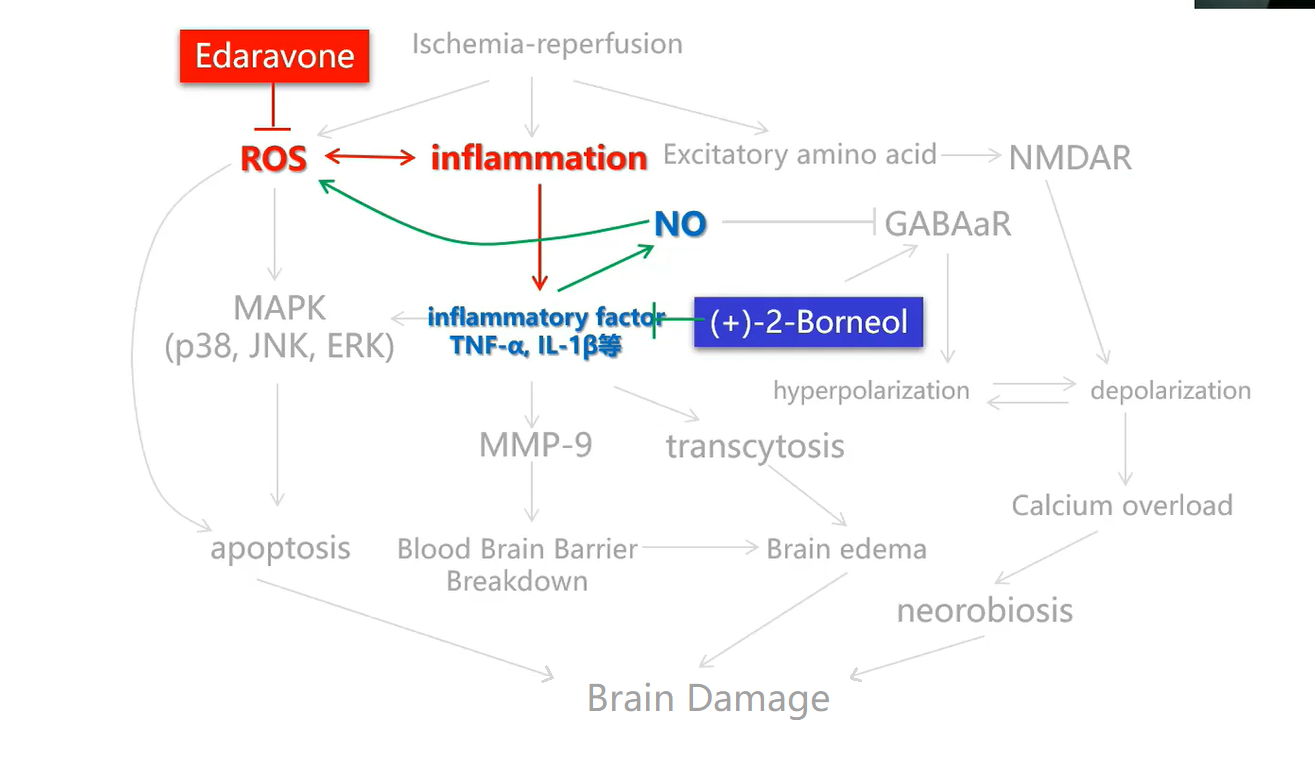
Sanbexin® (edaravone and dexbornol) is an innovative dual-mechanism neuron protection therapy independently developed by Simcere. The results of a phase III clinical trial (the TASTE study) published inSTROKE in 2021 has shown that, compared to edaravone monotherapy, Sanbexin® can significantly improve neurological symptoms and dysfunction of AIS patients, when the treatment starts within 48 hours of onset.
Since its launch in China in 2020, Sanbexin® has helped nearly 600,000 AIS patients, covering more than 2,700 hospitals nation-wide, and accumulating abundance of clinical experience for the multi-targeted neuron protection agent. The recently initiated TASTE-II study is expected to provide additional high-quality evidence and guidance for the clinical use of Sanbexin®, so as to benefit more Chinese AIS patients.