The annual meeting of the American Society of Clinical Oncology (ASCO) 2023, one of the most prestigious clinical academic events in oncology, is being held in Chicago, USA on June 2-6. Simcere Zaiming, an innovative oncology-focused biopharmaceutical company and a subsidiary of Simcere Pharmaceutical Group, exhibited a booth at ASCO for the first time. The Zaiming team held on-site meetings with clinicians from around the world to discuss the progress being made by the company by showcasing the latest he research and development of the company’s innovative anti-tumor drugs.

Bijoyesh Mookerjee,M.D., CMO of Simcere Zaiming(left 4)held pleasant conversation with visitors at Zaiming’s booth
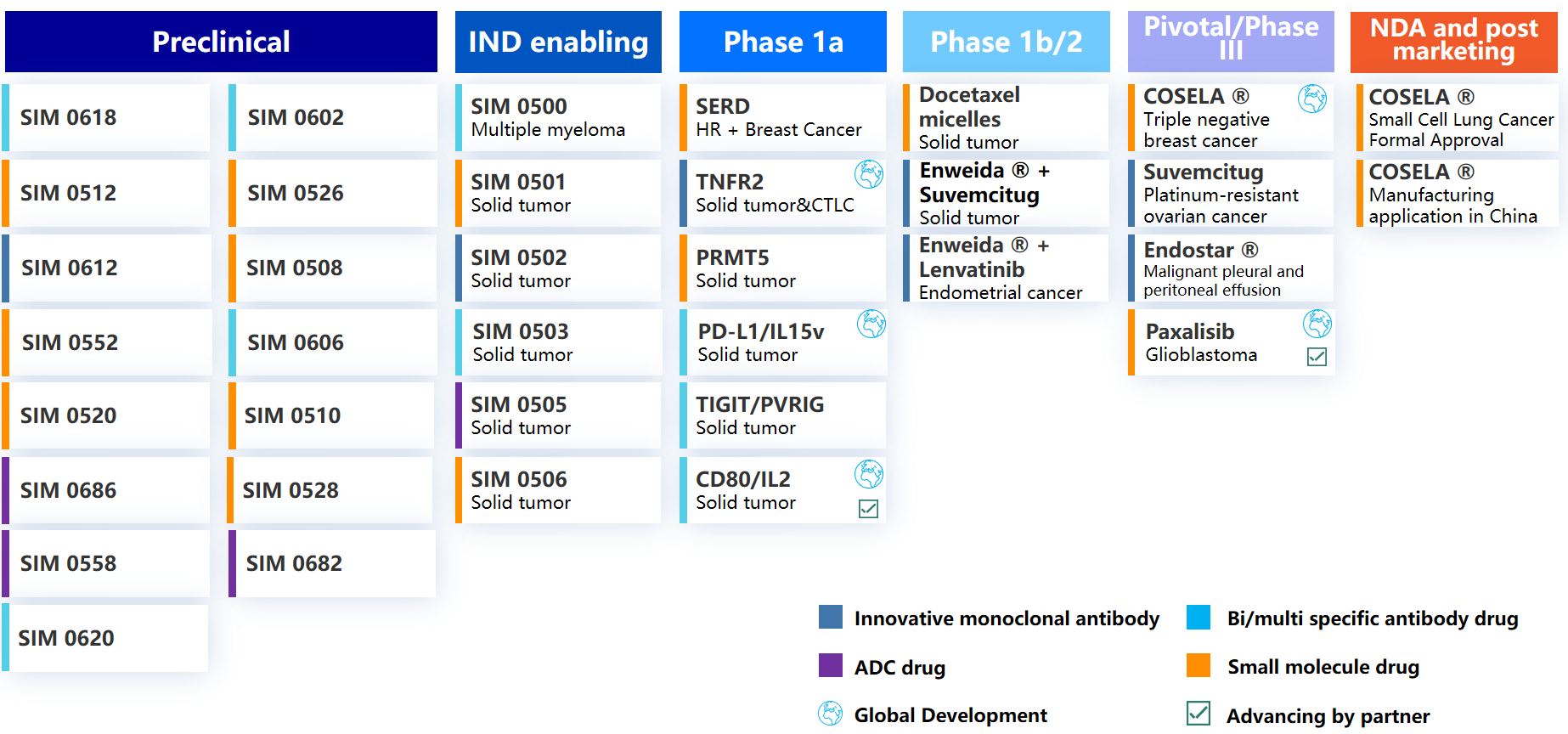
Zaiming showcased the company’s innovative R&D pipeline at ASCO Booth
A total of six relevant studies regarding three innovative drugs in Zaiming’s product portfolio, COSELA ®, Enweida ® and Endostar ® , have published the latest clinical progress in the form of posters and abstracts. Among them, there are clinical trials initiated by Chinese and foreign investigators (IIT), real-world studies (RWS), and registration studies promoted by Zaiming collaborative innovation partners, involving small cell lung cancer, non-small cell lung cancer, triple-negative breast cancer, gastric cancer, soft tissue sarcoma, and other cancer types. Congratulations to the Zaiming team!
COSELA® (trilaciclib) is an innovative bone marrow protection drug to be used prior to chemotherapy, developed by Simcere Pharmaceutical Co., Ltd. in cooperation with G1 Therapeutics of the United States. COSELA has been approved for marketing in China and the United States. Simcere Zaiming has the rights and interests of all indications of this product in Greater China
01 Real world outcomes of trilaciclib in ES-SCLC
Abstract No.: e20637
Author: Joseph Elijah et al., Roswell Park Comprehensive Cancer Center
Among the 78 patients with extensive-stage small cell lung cancer, 34 patients received trilaciclib before receiving the first-line PEA regimen. Even though there was a numerically lower rate of SN (3%) and hospitalization due to febrile neutropenia (FN) or infection (6%) in the PEAT versus the PEA (18%;11%) cohort, statistical significance was not met (p = 0.07;p = 0.065). Incidence of FN, platelet transfusion requirements, all-cause chemotherapy reductions, and treatment delays were not statistically different. However, the PEAT cohort as compared to PEA experienced a statistically significant reduction in red blood cell transfusion requirements (3% vs 23%; p = 0.02) and grade 3-4 anemia (6% vs 25%; p = 0.03). PFS and OS between the two cohorts were not statistically different.Results of this study support the integration of trilaciclib with PEA for ES-SCLC.
02 Neoadjuvant single-dose trilaciclib prior to combination chemotherapy in patients with early triple-negative breast cancer: Safety, efficacy, and immune data from a phase 2 study
Abstract No.: 603
Poster No.: 433
Author: Jeremy Meyer Force et al., Duke University School of Medicine
This clinical phase II single-arm open-label study (NCT05112536) used a single dose of trilaciclib as neoadjuvant therapy given to 24 patients with early-stage triple-negative breast cancer (TNBC), followed by trilaciclib + intensive standard chemotherapy (AC/T) regimen, and the appropriate use of immunotherapy or carboplatin. Each patient will receive radical surgery, the main purpose of the study is to explore the mechanism of action based on immunity after a single dose of trilaciclib. Response data were available for 15 pts; pathologic complete response (pCR) was observed in 7 (47%) pts, with pCR rates of 86% vs 13% for pts with PDL1+ vs PDL1– tumors, respectively. Immune analysis of the TME post trilaciclib monotherapy demonstrated several pCR-related features, such as increased stromal tumor-infiltrating lymphocytes and granzyme B+ cells. Correlative blood analysis revealed increased circulating baseline CD8+ T cells in pts achieving pCR. Safety and tolerability data are encouraging for trilaciclib in combination with AC/T ± pembrolizumab ± carboplatin in the neoadjuvant setting for early-stage TNBC. Preliminary efficacy data align with standard neoadjuvant chemotherapy regimens.
Enweida ® (Envafolimab) is an innovative subcutaneous injection PD-L1 antibody tumor drug from Simcere Pharmaceutical Group in strategic cooperation with 3Dmed and Alphamab, and Simcere Zaiming is responsible for the exclusive commercial promotion of the product in mainland China

01 First-line envafolimab plus SOX chemotherapy for PD-L1 positive metastatic or recurrent gastric adenocarcinoma: A multi-centre, single-arm phase II clinical trial.
Abstract No.: e16029
Author: Zhu Liangjun, Jiangsu Cancer Hospital
This multi-centre, single-arm, open-label study was conducted to evaluate the efficacy and safety of Envafolimab in metastatic or recurrent gastric adenocarcinoma. At present, eight patients were included for efficacy analysis, and nine for safety evaluation. The ORR was 50%, and DCR was 87.5%, based on RECIST v1.1. Survival data are not mature at present. The most common TRAEs were elevated AST (75%), elevated ALT (65%) and leukopenia (42.5%). No treatment-related deaths occurred. Envafolimab plus chemotherapy is a promising and tolerable therapeutic regimen for patients with metastatic or recurrent gastric adenocarcinoma.
02 ENVASARC: A pivotal trial of envafolimab and envafolimab in combination with ipilimumab in patients with advanced or metastatic unpleomorphic sarcoma or myxofibrosarcoma who have progressed on prior chemotherapy.
Abstract No.: TPS11583
Poster No.: 515a
Author: Richard F. Riedel et al. Duke Cancer Institute, Duke University Medical Center
ENVASARC (NCT 04480502) is a pivotal multicenter (at 30 U.S. and U.K. centers) open-label, randomized, non-comparative, parallel cohort study of envafolimab or envafolimab combined with ipilimumab in patients with locally advanced, unresectable or metastatic UPS/MFS with disease progression on one or two lines of prior therapy. The primary objective of each of parallel cohort is to demonstrate an ORR with a lower limit of the 95% confidence interval that excludes 5.0% in each cohort. If ≥ 9 responders are observed among the 80 patients enrolled in each cohort, then the lower bound of the 95% confidence interval will exclude 5.0%. Secondary endpoints include duration of response, PFS and OS. Key inclusion criteria: ≤ 2 prior lines of therapy ([neo]adjuvant therapy excluded), ECOG ≤ 1. Clinical trial information: NCT04480502
Endostar ® (recombinant human endostatin) is a Simcere and then Lumenis product, a angiogenesis inhibitor

01 Endostatin in combination with PD-1 antibody plus chemotherapy as first-line regimen for EGFR/ALK-negative, advanced or metastatic non-small cell lung cancer: A real-world study
Abstract No.: e20564
Author: Zhang Guoqing, et al., First Medical Center, Chinese PLA General Hospital
68 patients were included. The median PFS and OS were 22.0 months (95% confidence interval [CI]: 16.6-27.4 months) and 31.0 months (95% CI: 23.4 months-not reached [NR]), respectively, with a median follow-up of 21.4 months (range, 8.3-44.4 months). At the data cutoff, 33 patients (48.5%) remained progression-free and 24 patients (35.3%) had died. Short-term clinical efficacy was assessed after 2 consecutive treatment cycles, with an ORR of 72.06% (complete response [CR], n = 3; partial response [PR], n = 46) and a DCR of 95.59% (CR, n = 3; PR, n = 46; stable disease, n = 16). Further subgroup analysis determined that a significantly better ORR (89.2% vs. 51.6%), median PFS (23.4 vs. 13.2 months), and median OS (NR vs. 18.0 months) for patients with stage Ⅲ B/Ⅲ C vs. stage Ⅳ NSCLC (all p≤ 0.001). Besides, the median PFS (8.0 months vs. 22.5 months, p = 0.004) and OS (13.0 vs. 31.0 months, p = 0.027) of patients with brain metastasis were significantly shorter than those without brain metastasis. The ORR in patients who had a PD-L1 tumor proportion score (TPS) of < 1%, 1%-49%, ≥ 50%, and unknown were 50%, 50%, 75.0%, and 86.1%, respectively (p = 0.025). In the real-world scenario, endostatin combination with PD-1 antibody plus chemotherapy obtained encouraging efficacy and favorable safety in advanced or metastatic NSCLC.
02 Immunotherapy combined with rh-endostatin improved clinical outcomes over ICIs plus chemotherapy for second-line treatment of advanced NSCLC.
Abstract No.: e21133
Author: Hongxiang Huang et al., Department of Oncology, the First Affiliated Hospital of Nanchang University
The study retrospectively compared the effectiveness and safety of ICIs plus rh-endostatin to ICIs plus chemotherapy in patients with advanced non-small cell lung cancer (NSCLC). The median PFS with immunotherapy plus rh-endostatin (IE) was 7.10 months (95% CI, 4.64 to 9.56) versus 5.13 months (95% CI, 4.29 to 5.97) with immunotherapy plus chemotherapy (IC) (HR, 0.56; 95%CI, 0.33 to 0.95). Treatment-related adverse events of grade 3 or 4 occurred in 7.5% of the IE group versus 25.0% of the IC group. The ORR in the IE group was 35.0% versus 20.8% in the IC group (P = 0.137), and the DCR in the IE group was 92.5% versus 77.1% in the IC group (P = 0.049). The 1-year survival rate for the IE group was 69.4%, which was higher than the IC group's rate of 61.4%. ICIs in conjunction with endostatin therapy, demonstrate high efficacy and safety, suggesting that such a combination of therapy may be a viable treatment option for pretreated NSCLC patients in the future.
About Simcere Zaiming
Simcere Zaiming is a pharmaceutical company specializing in oncology, and a subsidiary of Simcere Pharmaceutical Group Limited, that focuses on the R&D, production and commercialization of innovative cancer therapeutics. The company was formed in 2023 and is committed to solving unmet clinical needs for cancer patients in China and around the world by developing breakthrough treatments. Simcere Zaiming has an innovative R&D pipeline with differentiated clinical value, among which 15 assets are currently in clinical trials. In addition to the company’s R&D portfolio, Simcere Zaiming is marketing three innovative drugs, COSELA®, Endostar®, and Envafolimab®. By collaborating with partners globally, Simcere Zaiming strives to bring potentially new innovative therapeutics to cancer patients worldwide.
Media Contact: pr@zaiming.com
About Simcere
Simcere Pharmaceutical Group Limited (2096.HK) is an innovation and R&D-driven pharmaceutical company. The company focuses on three therapeutic areas, oncology, central nervous system and autoimmune diseases, with a forward-looking vision toward disease areas that may have significant clinical needs in the future, aiming to achieve the mission of "providing today's patients with medicines of the future." Leveraging its R&D capability and commercialization excellence, Simcere has built a market-leading product portfolio in China. Its vigorous in-house R&D efforts and extensive R&D collaborations have made it a strategic cooperation partner with world leading innovative companies and research institutes.
Media contact: simcere.mediarelations@simcere.com