On April 1, Simcere Pharmaceutical Group (2096. HK) announces that the Phase 2 clinical study of Docetaxel Polymeric Micelles in patients with early solid tumors has completed the first patient enrollment (FPI) in Tianjin Cancer Hospital.
This open-label, multi-cohort, multi-center phase II study led by Professor Guo Ye from Shanghai Tongji University Affiliated Oriental Hospital aims to assess the safety and tolerability of the docetaxel polymeric micelles based treatment regimen in patients with advanced esophageal cancer, and to evaluate the antitumor efficacy of docetaxel polymeric micelles in patients with advanced solid tumors.
Docetaxel is a classic antitumor chemical drug and one of the backbone chemotherapy widely used in solid tumor treatment regimens. However, problems of poor stability, allergic reactions and toxic side effects often arise with the dosage form and pharmaceutical excipients of docetaxel injection products currently marketed in China.
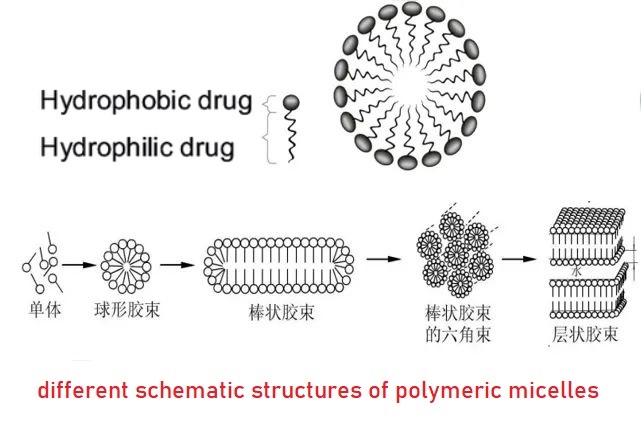
Docetaxel polymeric micelles is an innovative dosage form co-developed by Simcere and Suzhou Hightechbio Biotechnology Co., Ltd. The polyethylene glycol monomethyl ether-polylactic acid block copolymer (mPEG-PDLLA), an amphiphilic biocompatible biodegradable material, is used as the solubilizing carrier of docetaxel to reduce the allergy and hematotoxicity of docetaxel injection to facilitate clinical application.
The results of the phase I clinical trial showed that, docetaxel polymeric micelles was well tolerated by patients within the dosage range of 25mg/m2~100mg/m2. Among 7 subjects in the 75mg/m2 dose group , there were 2 cases of Partial Remission(PR) and 2 cases of Stable Disease(SD). 5 cases in the 100 mg/m2 dose group evaluated for curative effect reached Stable Disease.
Simcere is working closely with clinical experts to accelerate the development of docetaxel polymeric micelles, in hope of offering new options to cancer patients soon with improved patient tolerability, safety and therapeutic effect than convention docetaxel injections.